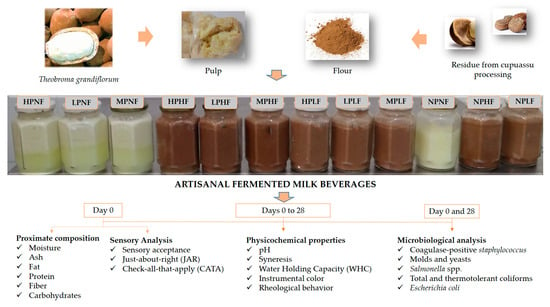
3.3. Rheological Behavior
The behavior of the fermented dairy beverages with added cupuassu pulp and flour was described using the Ostwald–de Waele model, as reported in
Table 5. This model relates the shear stress to the shear rate of a fluid [
33]. According to Moreira et al. [
55], it provides better adjustment to the experimental data for whey-based products, similar to that reported herein. The values obtained for the correlation coefficient in the different samples were greater than 0.910 (R
2 ≥ 0.910), which indicates that the power law model provided suitable adjustment parameters. The consistency index (K) gives an idea of the fluid viscosity; the K values were generally unaffected by flour alone compared with the control, regardless of the added concentration (
p > 0.05). On the other hand, the medium and high pulp concentrations elevated the K values, while the low concentration was insignificant. Similarly, 10% and 7.5% pulp in combined treatments provided higher consistency indexes than the control, regardless of flour concentration. In contrast, 5% pulp generally did not affect K, regardless of the combined flour concentration. The addition of pulp presented a positive correlation with K. Pulp acidified the pH and reduced syneresis, mainly when at a higher concentration. Accordingly, these factors contributed to increased K, as reported by the inverse correlation between them (
Table S1). However, increased WHC obtained by adding pulp also favored higher consistency (
p = 0.03;
Table S1).
At the end of storage, despite fluctuations, the control and the treatments with flour alone and with pulp alone at 10% had K values similar to those of the freshly prepared product. In contrast, pulp alone at the medium and low concentrations progressively increased the consistency index. The behavior was varied for combined treatments with 3% flour; HPLF and MPLF had lower and higher final values than the control, respectively, while LPLF was similar to the control after 28 days in terms of values. In combined treatments with 1.5% flour, the medium and low pulp concentrations elevated K during storage, while the high pulp concentration resulted in K values similar to those of the control. These differences in behavior can be attributed to the different effects of treatments on pH, syneresis, and WHC during storage (
Table S1).
Table 5.
Rheological parameters obtained with Ostwald–de Waele model of artisanal fermented milk beverages.
Table 5.
Rheological parameters obtained with Ostwald–de Waele model of artisanal fermented milk beverages.
| Parameter | Sample | Storage Time (Days) |
|---|
| 0 | 7 | 14 | 21 | 28 |
|---|
| K (mPa·sn) | NPNF | 230.59 ± 41.49 efC | 208.88 ± 35.18 gC | 351.11 ± 17.01 eAB | 402.64 ± 14.38 dA | 283.49 ± 65.70 efBC |
| NPLF | 161.89 ± 1.94 fC | 198.50 ± 47.44 gBC | 385.46 ± 39.57 eA | 290.75 ± 49.20 eAB | 235.03 ± 2.51 fBC |
| NPHF | 413.84 ± 47.67 cdA | 166.83 ± 15.96 gC | 306.27 ± 65.92 eB | 388.41 ± 20.70 dAB | 357.08 ± 24.35 eAB |
| LPNF | 174.29 ± 44.94 efD | 252.67 ± 46.45 fgCD | 365.38 ± 5.42 eBC | 449.35 ± 39.63 dAB | 530.60 ± 61.26 dA |
| MPNF | 406.37 ± 19.27 cdD | 368.59 ± 30.99 deD | 543.88 ± 11.13 cdC | 774.28 ± 1.07 bB | 1284.53 ± 57.90 aA |
| HPNF | 556.90 ± 1.95 cA | 574.04 ± 54.25 abA | 650.88 ± 38.77 bA | 640.47 ± 22.88 cA | 563.23 ± 44.25 dA |
| HPLF | 1699.10 ± 146.40 aA | 502.12 ± 23.98 bcC | 663.73 ± 13.83 bC | 944.51 ± 50.64 aB | 530.26 ± 23.16 dC |
| MPLF | 390.43 ± 63.37 dC | 625.36 ± 43.18 aB | 581.54 ± 15.67 bcB | 625.30 ± 52.86 cB | 949.46 ± 7.44 bA |
| LPLF | 176.59 ± 2.52 efC | 213.04 ± 50.82 gC | 331.69 ± 27.51 eAB | 407.97 ± 14.13 dA | 253.21 ± 32.37 efBC |
| HPHF | 843.50 ± 5.47 bA | 473.90 ± 38.64 bcdC | 769.92 ± 26.69 aB | 849.56 ± 16.26 abA | 797.68 ± 27.82 cAB |
| MPHF | 477.13 ± 34.40 cdC | 453.05 ± 35.28 cdeC | 490.73 ± 3.52 dC | 664.53 ± 16.36 cB | 926.42 ± 19.85 bA |
| LPHF | 331.82 ± 36.22 deB | 356.20 ± 16.87 efB | 313.91 ± 13.26 eB | 449.45 ± 37.20 dA | 496.54 ± 4.63 dA |
| n | NPNF | 0.438 ± 0.03 bcdA | 0.461 ± 0.07 abA | 0.398 ± 0.03 bcA | 0.382 ± 0.02 abA | 0.426 ± 0.05 bcA |
| NPLF | 0.458 ± 0.02 bcA | 0.454 ± 0.04 abA | 0.333 ± 0.01 deB | 0.405 ± 0.03 aA | 0.444 ± 0.01 abA |
| NPHF | 0.385 ± 0.02 deB | 0.450 ± 0.02 abA | 0.398 ± 0.04 bcAB | 0.349 ± 0.02 bcdB | 0.379 ± 0.05 cdB |
| LPNF | 0.488 ± 0.05 abA | 0.479 ± 0.03 aA | 0.417 ± 0.01 abAB | 0.351 ± 0.02 bcdB | 0.363 ± 0.02 deB |
| MPNF | 0.350 ± 0.01 efB | 0.416 ± 0.02 abA | 0.359 ± 0.01 cdB | 0.309 ± 1 × 10−3 dC | 0.229 ± 2 × 10−3 gD |
| HPNF | 0.348 ± 0.01 efB | 0.383 ± 0.01 bcA | 0.378 ± 2 × 10−3 bcdA | 0.375 ± 3 × 10−3 abA | 0.383 ± 0.01 cdA |
| HPLF | 0.297 ± 0.01 fC | 0.401 ± 0.01 abA | 0.349 ± 2 × 10−3 deB | 0.312 ± 0.01 cdC | 0.401 ± 0.02 bcdA |
| MPLF | 0.397 ± 0.30 cdeA | 0.310 ± 0.01 cB | 0.307 ± 0.01 eB | 0.342 ± 0.01 bcdB | 0.308 ± 0.01 fB |
| LPLF | 0.534 ± 0.01 aA | 0.458 ± 0.04 bcA | 0.413 ± 0.02 abCD | 0.384 ± 0.01 abD | 0.482 ± 0.02 aAB |
| HPHF | 0.359 ± 0.03 efB | 0.434 ± 0.02 abA | 0.343 ± 3 × 10−3 deB | 0.348 ± 2 × 10−3 bcdB | 0.360 ± 0.01 defB |
| MPHF | 0.375 ± 0.01 deB | 0.427 ± 1 × 10−3 abA | 0.371 ± 3 × 10−3 bcdB | 0.358 ± 0.01 abcC | 0.322 ± 1 × 10−3 efD |
| LPHF | 0.402 ± 0.01 cdeBC | 0.412 ± 0.02 abAB | 0.457 ± 0.01 aA | 0.360 ± 0.03 abcC | 0.382 ± 0.01 cdBC |
| Apparent viscosity (mPa∙s) | NPNF | 57.12 ± 15.78 efB | 49.50 ± 19.00 eB | 98.06 ± 12.77 fgAB | 118.04 ± 12.10 efA | 74.05 ± 27.57 fAB |
| NPLF | 37.10 ± 3.04 deC | 47.34 ± 17.20 eBC | 132.32 ± 19.19 efA | 80.17 ± 20.10 fB | 56.35 ± 1.49 fBC |
| NPHF | 119.56 ± 6.17 efA | 39.39 ± 5.72 eB | 87.07 ± 29.14 gA | 126.60 ± 14.28 efA | 105.60 ± 12.57 efA |
| LPNF | 37.45 ± 15.04 eD | 54.82 ± 14.61 deCD | 95.50 ± 2.54 fgBC | 146.00 ± 22.78 deAB | 165.7 ± 31.10 dA |
| MPNF | 131.90 ± 8.56 deCD | 97.25 ± 15.15 cdD | 171.07 ± 5.73 cdC | 286.56 ± 2.23 bB | 614.21 ± 32.71 aA |
| HPNF | 181.54 ± 3.04 cdA | 167.47 ± 18.40 bA | 191.70 ± 13.36 bcA | 191.42 ± 7.67 cdA | 164.28 ± 17.93 dA |
| HPLF | 655.25 ± 77.48 aA | 138.27 ± 11.04 bcC | 216.09 ± 7.25 bC | 345.76 ± 24.14 aB | 146.11 ± 12.68 deC |
| MPLF | 110.35 ± 28.32 bcC | 230.93 ± 21.90 aB | 216.52 ± 9.52 bB | 208.23 ± 24.42 cB | 352.67 ± 5.87 bA |
| LPLF | 31.63 ± 1.27 fC | 49.95 ± 17.31 eC | 88.01 ± 12.18 gB | 118.46 ± 3.33 efA | 54.01 ± 10.27 fC |
| HPHF | 266.20 ± 19.98 bA | 117.42 ± 15.169 cB | 255.02 ± 5.60 aA | 277.42 ± 7.27 bA | 250.37 ± 10.14 cA |
| MPHF | 142.52 ± 8.35 deC | 114.67 ± 8.48 cD | 148.76 ± 3.13 deC | 209.97 ± 5.37 cB | 328.24 ± 8.78 bA |
| LPHF | 91.18 ± 12.57 efB | 94.87 ± 10.05 cdB | 72.34 ± 6.04 gB | 142.43 ± 25.40 eA | 145.34 ± 3.42 deA |
The n index indicates the degree of deviation from the Newtonian flow (n = 1). As n values were ≤ 0.534 (
Table 5), all samples presented a non-Newtonian behavior. It indicates that the beverages had the typical performance of pseudoplastic fluids, which is characteristic of this type of dairy product [
56,
57]. This behavior is corroborated by
Figure S2, where the shear stress increased as a function of the shear rate in all samples, regardless of storage time. The values of n were not affected by the addition of pulp alone at the low concentration compared with the control (
p > 0.05), while the medium and high concentrations of pulp decreased the values of n (
p < 0.05). On the other hand, the addition of flour did not generally affect the values of n, regardless of the added concentration (
p > 0.05). Differently combined samples with the high pulp concentration provided lower consistency indices than the control, regardless of flour concentration, while samples with the medium pulp concentration showed no significant difference, regardless of flour concentration, as did LPHF (containing 5% pulp and 3% flour) in contrast to LPLF (containing the same concentration of pulp but less flour), with which the index was significantly higher (
p < 0.05).
During storage, the control showed no significant difference (
p > 0.05); in contrast to the samples with only flour and only pulp, LPNF and MPNF decreased n values, while HPNF increased with time, indicating that storage time, pulp addition, and pH favored n (
Table S1). The HPLF and MPHF combined samples showed an increase in n on day 7 of storage and a subsequent decrease until days 21 and 28, respectively. The value of n in MPLF decreased during storage, while that in LPLF only decreased after day 7. HPHF and LPHF presented the highest values on days 7 and 14, respectively. Consistently, K was a strong predictor of n (R
v = 0.786;
p < 0.001;
Table S1); as the K values increased, the n values decreased (
Figure 7a).
Apparent viscosity describes the flow behavior, an important parameter that influences sensory attributes and quality in dairy beverages, since the addition of whey generates low viscosity in this type of product [
57].
Table 5 presents the values obtained for viscosity; it was observed that the addition of pulp at the highest concentration increased the apparent viscosity compared with the control (
p < 0.05), while flour did not generate a significant effect, regardless of concentration (
p > 0.05). On the other hand, the combination of flour and pulp ingredients in the LPLF, MPHF, and LPHF samples did not show significant changes compared with the control (
p > 0.05), in contrast to HPLF, MPLF, and HPHF, which showed a significant increase in apparent viscosity values (
p > 0.05).
During storage, the control sample showed an increase in viscosity on days 21 and 28; this behavior was also present in the samples with 1.5% flour, while the sample with 3% flour only showed a significant variation on day 7, obtaining the lowest value (
p < 0.05). Samples with only-pulp addition increased viscosity over time, which could be attributed to the reduction in pH due to the addition of pulp favoring higher apparent viscosity (
p = 0.04;
Table S1). Samples combined with the lowest pulp concentration (LPLF and LPHF) increased apparent viscosity values on days 21 and 28, respectively (
p < 0.05). However, LPLF showed no significant difference from the control on day 28 (
p > 0.05). Concerning the samples combined with the medium pulp concentration (MPHF and MPLF), they showed a decrease on day 7, where MPHF subsequently increased until day 28 (
p < 0.05), showing a higher apparent viscosity value than the control. In general, HPLF and HPHF samples had the highest apparent viscosity at the beginning of storage (
p < 0.05). However, these had the lowest values on day 7, which can be attributed to the decrease in n during storage and the increase in K caused by the addition of pulp, which positively affected the viscosity (
Figure 7b;
p < 0.001;
Table S1).
Figure 7.
Significant correlations (p < 0.05) internally validated with the bootstrap method (a) between K and n and (b) between K and apparent viscosity in artisanal fermented milk beverages stored at 4 °C.
Figure 7.
Significant correlations (p < 0.05) internally validated with the bootstrap method (a) between K and n and (b) between K and apparent viscosity in artisanal fermented milk beverages stored at 4 °C.
3.5. Sensory Analysis
The results of the acceptability test and purchase intention of the artisanal fermented milk beverages with cupuassu flour and pulp are shown in
Table 6 and
Figure S3. Formulations with 1.5% flour tended to present lower sensory scores than formulations with 3% flour regarding appearance, color, consistency, and firmness. Thus, MPLF and LPLF scored lower than HPHF, while only HPLF scored lower than MPHF and LPHF in appearance. In terms of color, HPHF was superior to all treatments with 1.5% flour, while MPHF and LPHF scored higher than MPLF and LPLF. MPLF scored lower than all treatments with 3% flour in consistency, and it scored lower than HPHF and LPHF in firmness. The high flour concentration positively affected the appearance (R
v = 0.825;
p = 0.032;
Table S2), color (R
v = 0.936;
p = 0.005;
Table S2), and flavor (R
v = 0.803;
p = 0.039) of the artisanal fermented milk beverages, which was attributed to the decrease in syneresis caused by the increase in WHC, which increased the scores in appearance and color attributes (
Figure S4A–D), as well as color and carbohydrate content typical of cupuassu flour [
19]. On the other hand, flour concentration was not relevant to flavor, taste, overall acceptability, and purchase intention.
Additionally, in treatments with 1.5% flour, higher pulp content improved taste, consistency, and purchase intention scores compared with average pulp levels. Indeed, HPLF scored higher than MPLF in these sensory attributes. The results indicate that the 10% pulp concentration could have a positive sensory effect on artisanal fermented milk beverages with 1.5% flour. This acceptance could be attributed to the content of ascorbic acid and phenolic compounds that pulp contains. Together with the volatile substances of fermented beverages, they provide better odor and flavor to the product [
44]. In terms of purchase intention, the low valuations obtained by the evaluated samples (2.13 to 2.71) were close to the term “maybe buy, maybe not buy”; this could be attributed to the strong flavor that cupuassu pulp has and the lack of familiarity with the consumption of this type of fruit [
13,
21].
Table 7 shows the values obtained on the JAR scale, used to identify the optimum intensity of each of the attributes of the artisanal fermented milk beverages. The parameters of aroma (acid, alcoholic, cupuassu, and milk), color (white and beige), flavor (caramel), and texture (sandiness, consistency, viscosity, and mouthfeel) showed no significant difference (
p > 0.05) among the samples. However, pulp concentration was a strong predictor of an alcoholic aroma (R
v = 0.837;
p = 0.034;
Table S2), which, at the same time, had an inverse correlation with the
b* color parameter (yellowness), that is, the higher the perception of
b* was, the lower the alcoholic aroma was (R
v = 0.875;
p = 0.020;
Table S2; Figure S5A). In the samples, pH was highly predictive of a cupuassu aroma (R
v = 0.985;
p = 0.007;
Table S2), which was generated by the decrease in pulp and the increase in flour in the beverage (
Figure S5B). Furthermore, with the concentration of 1.5% flour, a higher percentage of pulp helped to reduce the bitterness (HPLF < LPLF;
p < 0.05).
Pulp could also contribute to elevate cupuassu flavor, but this was only significant at the concentration of 3% flour (HPHF > LPHF;
p < 0.05). On the other hand, flour concentration contributed to a less sweet taste but a more acidic and bitter taste. Indeed, the acidic score of MPHF was higher than those of HPLF and LPLH. In terms of bitter taste, the scores of HPHF and MPHF were superior to that of HPLF. In contrast, MPHF and LPHF had sweet taste scores that were lower than those of LPLF and HPLF. This pattern was attributed to the correlation between flour concentration and bitter taste, where flour addition was a strong predictor of bitter taste (R
v = 0.928;
p = 0.006;
Table S2), which was also positively affected by WHC and reduced syneresis (
Figure S5C,D).
Flour also favored the darkening of the beverages. Flour increased brown color perception scores and decreased white color perception scores (
p = 0.002;
Table S2), such that all treatments with 3% flour had higher browning scores than MPLF and LPLF, which was attributed to decreased syneresis and increased WHC (
Figure S6A–D). However, flour did not influence the score obtained in cupuassu flavor (
p > 0.05), unlike the pulp percentages, which generated increases in
L* and
C* color parameters, favoring cupuassu flavor (
p = 0.036;
Table S2) with the increase in pulp percentage (
Figure S6E,F).
In terms of texture, the firmness attribute was the one that showed variations among samples (
p < 0.05) but no correlation concerning pulp and flour concentration (
Table S2), in contrast to the mouthfeel attribute, which was favored by apparent viscosity, resulting from consistency index K, and the addition was inversely related to the behavior of
a* (
Figure S7A–C). The results indicate that 3% cupuassu flour could increase the acid and bitter taste, brown color, and firm texture of the artisanal fermented milk beverages. Likewise, adding 1.5% cupuassu flour increased the sweet taste values.
According to the results, a penalty analysis of the JAR scores was conducted (
Table 8) to improve the formulations of the artisanal fermented milk beverage by adding cupuassu pulp and flour. Parameters with penalty score > 0.5 and incidence > 20% were considered attributes detrimental to overall acceptability. All treatments were penalized for excessive acid aroma, acid taste, and bitter taste, as well as for lack of milk aroma, sweet taste, caramel taste, consistency, firmness, viscosity, and mouthfeel.
Samples HPHF, MPHF, HPLF, and MPLF with 10% or 7.5% pulp were penalized for an excessive alcoholic aroma, which may be because cupuassu pulp represents an important source of substrates for the group of microorganisms (yeasts, lactic, and acetic acid bacteria) of the cupuassu microbiota, which are involved in alcoholic fermentation [
47]. Accordingly, MPHF was penalized for an excessive cupuassu aroma, while LPHF and LPLF were penalized for the lack of this aroma. Similarly, HPHF, MPHF, and MPLF presented excessive cupuassu flavor, contrary to LPHF (5% pulp and 3% flour), which was penalized for lack of flavor. This indicates that the ideal pulp concentration has yet to be achieved. Samples with 1.5% flour (HPLF, LPLF, and MPLF) were penalized for lacking brown color. For the brown color attribute, 3% flour was a more promising concentration.
However, it is important to note that a higher flour concentration increased acidity and bitterness, which were penalized attributes. Thus, an ideal flour concentration to harmonize these effects must be established. LPHF, MPHF, and MPLF presented excessive sandiness. Despite the penalty results, most evaluated attributes were close to “moderately more than ideal” (JAR between 2.49 and 3.85). The exceptions were sweetness and caramel flavor, which were less than “ideal” (JAR between 1.74 and 2.28) in all treatments. This can be attributed to the low percentage of sugar in the beverage (3%). The combination of the JAR profile data and the penalty analysis made it possible to establish an ideal product with an adjustment of the percentages of cupuassu pulp and flour, increasing the percentage of sweetener in the product, which would also reduce the acid aroma, acid taste, and bitter taste.
Figure 8 shows the projection of the CATA data of the artisanal fermented milk beverage samples. It explains 94.59% of the total variation of the data, with F1 of 87.06% and F2 of 7.53%. According to the values obtained in the Cochran’s Q-test, seven terms were statistically different among the artisanal fermented milk beverages (
p < 0.05). These terms were homogeneous appearance, dark brown color, light brown, beige color, white color, bitter taste, and cocoa aroma. Samples containing 3% cupuassu flour (HPHF, MPHF, and LPHF) were described as having bitter taste, dark brown color, and homogeneous appearance. MPHF and LPHF were also associated with a cocoa aroma, which could be attributed to the aromatic compounds in the fermented cupuassu and cocoa seeds, which impart an identical aroma [
18].
On the other hand, samples containing 1.5% cupuassu flour (HPLF, MPLF, and LPLF) were described as white, light brown, and beige. These results showed similar behavior in the evaluators’ scores, for both CATA and JAR, where the samples with the highest concentration of cupuassu flour (3%) were perceived as having the most bitter taste and brown color. Likewise, the white and beige colors were associated with the samples with the lowest concentration of cupuassu flour (1.5%).